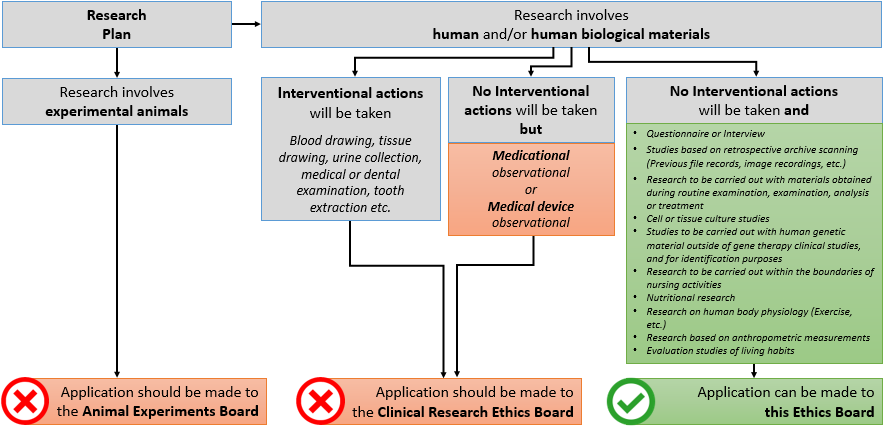
The Scope of Non-Interventional Clinical Research Institutional Review Board
Please read the scope of this board specified below if your research is qualified for submission.
- Studies that are planned to be carried out without requiring the direct intervention of a physician and without the need for any interventional procedure are taken into consideration.
| Studies Within the Scope of Evaluation |
| - All observational studies (EXCEPT medicational/drug observation studies and/or medical device observational studies) |
| - Survey studies |
| - Studies based on retrospective archive scanning (File records, image recordings, etc.) |
| - Research to be carried out with archive (collection) materials obtained previously during routine examination, examination, analysis or treatment |
| - Cell or tissue culture studies |
| - Studies to be carried out with human genetic material outside of gene therapy clinical studies, and for identification purposes |
| - Research to be carried out within the boundaries of nursing activities |
| - Nutritional research |
| - Research on human body physiology (Exercise, etc.) |
| - Research based on anthropometric measurements |
| - Evaluation studies of living habits |
Out of the Scope of Non-Interventional Clinical Research Institutional Review Board
- Studies involving interventional procedures are not taken into consideration.
- Interventional procedures such as blood sampling, tissue collection, saliva collection, urine collection, medical and/or dental examination, medical imaging, tooth extraction etc. are not considered within the scope of this board.